Remsima™ SC
-
Remsima™ SC is the world's first subcutaneous formulation of biosimilar infliximab developed by Celltrion. It was approved by Health Canada for rheumatoid arthritis on January 28, 2021, and for Crohn’s disease and ulcerative colitis on February 15, 2024.
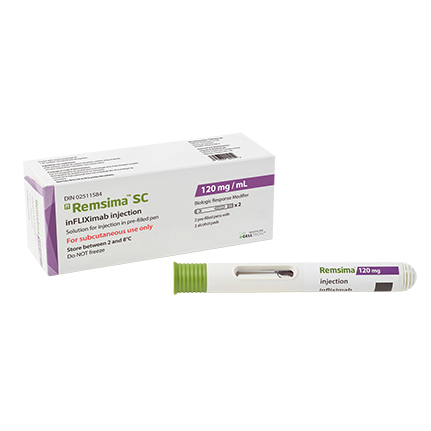
- Product Name : Remsima™ SC
- INN : Infliximab
- Indications : rheumatoid arthritis Crohn’s disease ulcerative colitis
- Protein Type : Monoclonal antibody (mAb)
- Mechanism of Action : It slows down the disease progression by neutralizing tumor necrosis factor-alpha (TNF-a), which is a common cause of autoimmune diseases.
- Drug Approval Status : Health Canada approved
*This product description is for medical and educational purposes only. They are not intended to promote PR or sales campaigns.
*For more details, please consult a doctor or a medical specialist.
*This site is intended for all over the world. -
Yuflyma® is a low volume/high concentration biosimilar of adalimumab available in 40 and 80mg strengths in both Auto injector and pre-filled syringe formats. It was approved by Health Canada on December 24, 2021.
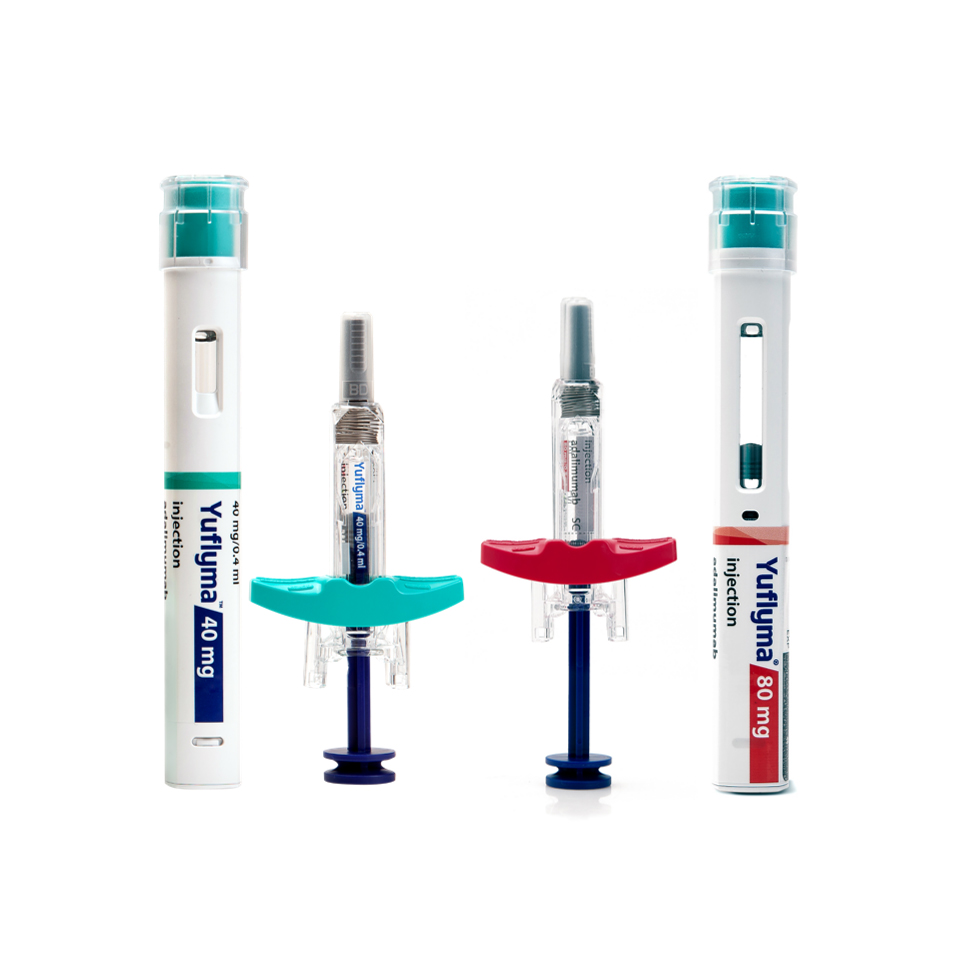
- Product Name : Yuflyma®
- INN : Adalimumab
- Indications : Rheumatoid arthritis Psoriatic arthritis Ankylosing spondylitis Psoriasis Adult Crohn’s disease Adult ulcerative colitis Polyarticular juvenile idiopathic arthritis Adult and adolescent hidradenitis suppurativa Adult and pediatric uveitis
- Protein Type : Monoclonal antibody (mAb)
- Strength : 40mg/0.4mL, 80mg/0.8mL
- Drug Approval Status :Health Canada approved
*This product description is for medical and educational purposes only. They are not intended to promote PR or sales campaigns.
*For more details, please consult a doctor or a medical specialist.
*This site is intended for all over the world. -
Vegzelma™ is a biosimilar of bevacizumab developed by Celltrion. It was approved by Health Canada on January 3rd, 2023.
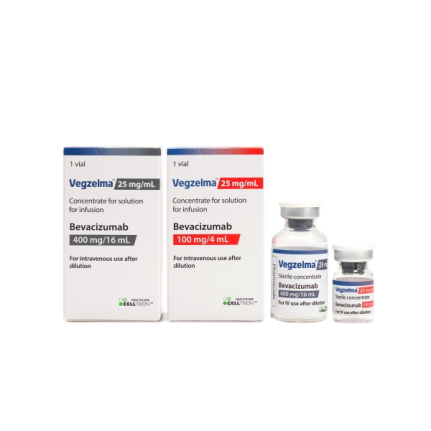
- Product Name : Vegzelma™
- INN : bevacizumab
- Indications : Metastatic Colorectal Cancer (mCRC) Locally Advanced, Metastatic or Recurrent Non-Small Cell Lung Cancer (NSCLC) Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube and Primary Peritoneal Cancer Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube and Primary Peritoneal Cancer Malignant Glioma (WHO Grade IV) – Glioblastoma
- Protein Type : Monoclonal antibody (mAb)
- Strength : 100mg/4mL, 400mg/16mL
- Drug Approval Status :Health Canada approved
*This product description is for medical and educational purposes only. They are not intended to promote PR or sales campaigns.
*For more details, please consult a doctor or a medical specialist. -
Steqeyma® is a biosimilar of ustekinumab developed by Celltrion. It was approved by Health Canada on July 30th, 2024.
- Product Name : Steqeyma®
- INN : ustekinumab
- Indications :
- Plaque Psoriasis - Psoriatic Arthritis - Crohn’s Disease
- Protein Type : Monoclonal antibody (mAb)
- Strength :
45mg/0.5mL, 90mg/1mL (SC) 130mg/26ml (IV) - Drug Approval Status :Health Canada approved
*This product description is for medical and educational purposes only. They are not intended to promote PR or sales campaigns.
*For more details, please consult a doctor or a medical specialist. -
Omlyclo™ is the world’s first and only omalizumab biosimilar developed by Celltrion. It was approved by Health Canada on December 6th, 2024.
- Product Name : Omlyclo™
- INN : Omalizumab
- Indications :
Allergic Asthma (adult and pediatric patients – 6 years of age and above) Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP) Chronic Idiopathic Urticaria (CIU)
- Protein Type : Monoclonal antibody (mAb)
- Strength :
IgE plays a central role in the pathophysiology of inflammatory diseases in the airway. Omalizumab binds to IgE and prevents binding of IgE to the high-affinity IgE Receptor, FcεRI, thereby reducing the amount of free IgE that is available to trigger the allergic-inflammatory cascade. - Drug Approval Status :Health Canada approved on December 6th, 2024
*This product description is for medical and educational purposes only. They are not intended to promote PR or sales campaigns.
*For more details, please consult a doctor or a medical specialist.